electron configuration of gd|Iba pa : Bacolod Electron configurationThe arrangements of electrons above the last (closed shell) noble gas. Melting pointThe temperature at which the solid–liquid phase change occurs. . Huwag palampasin ang saya, sumali na sa rebolusyon ng mobile gaming ngayon! . SUPERJILI 777 Win Rates: ⭐️⭐️⭐️⭐️⭐️100% Legit in the Philippines SUPERJILI 777: Your Ultimate Gaming Destination Introduction Welcome to the world of SUPERJILI 777, where online gaming reaches new heights. . PHTAYA06 | .
PH0 · outer electronic configuration of gd
PH1 · electronic configuration of gd 64
PH2 · electronic configuration of gadolinium
PH3 · Iba pa
Pinay insan kong gustong kantotin ng todo habang naka higa. Sheet sarap mo tumuwad Jean. Jackpot ang nene sa jumbo hotdog. Bullseye patalikod ang pinay hot babe. Isang round ng tsupaan bago matulog. Ligo muna sa round 1 para fresh sa round 2. Real sweet homemade kantotan talaga basta Afam.
electron configuration of gd*******The arrangement of electrons in gadolinium in specific rules in different orbits and orbitals is called the electron configuration of gadolinium. The electron configuration of gadolinium is 4f 7 5d 1 6s 2, if the electron .
electron configuration of gd Iba pa Mar 23, 2023 Electron configurationThe arrangements of electrons above the last (closed shell) noble gas. Melting pointThe temperature at which the solid–liquid phase change occurs. .
The chemical symbol for Gadolinium is Gd. Electron Configuration and Oxidation States of Gadolinium. Electron configuration of Gadolinium is [Xe] 4f7 5d1 .Gadolinium is the eighth member of the lanthanide series. In the periodic table, it appears between the elements europium to its left and terbium to its right, and above the actinide curium. It is a silvery-white, malleable, ductile rare-earth element. Its 64 electrons are arranged in the configuration of [Xe]4f 5d 6s , of which the ten 4f, 5d, and 6s electrons are valence.
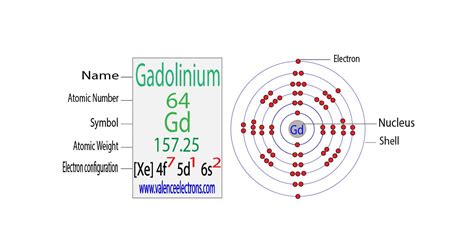
Electron configuration for gadolinium. The history of Gadolinium. Periodic table history. Identifiers. List of unique identifiers for Gadolinium in various chemical registry databases.electron configuration of gdElectron configuration for gadolinium. The history of Gadolinium. Periodic table history. Identifiers. List of unique identifiers for Gadolinium in various chemical registry databases.Electron configuration for Gadolinium (element 64). Orbital diagram. Gadolinium electron configuration. ← Electronic configurations of elements. Gd (Gadolinium) is .Typical Unstable Isotopes. Electrons and Electron Configuration. The number of electrons in an electrically-neutral atom is the same as the number of protons in the .

⬆. Get the facts about element Gadolinium (Gd) [64] from the periodic table. Find physical data, electron configuration, chemical properties, aggregation states, isotope data . Ground state gadolinium orbital diagram. Gd orbital diagram. Gadolinium 3+ electron configuration. The electronic configuration of Gd3+ is Xe54 4f7 5d0 6s0. .
The distribution of electrons into orbitals of an atom is called its electronic configuration. Electronic configuration of Gadolinium: The symbol for Gadolinium is Gd and has atomic number 64. The electronic configuration of gadolinium is Xe 4 f 7 5 d 1 6 s 2. Gd is an f-block element and belongs to the lanthanide series. Hence, the correct .
November 24, 2022 by Mansi Sharma. Gd, the chemical symbol for gadolinium, is the 64th element on the periodic table. Let us talk in more detail about this element’s electronic configuration. Electronic configuration of Xe54 4f7 5d1 6s2 having an atomic number 64 is assigned to gadolinium It is a rare-earth element, ductile, and malleable.The electronic configuration of Gd 2+ is (at. No. of Gd is 64) View Solution. Q2. The electronic configuration of bivalent europium and trivalent cerium, are: (Atomic Number : Xe = 54, Ce = 58, Eu = 63) View Solution. Q3. The electronic configuration of Eu 3+ ion is: View Solution. Q4.
How many protons, neutrons, and electrons are in atoms of these isotopes? Write the complete electron configuration for each isotope. Answer. Co has 27 protons, 27 electrons, and 33 neutrons: 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 7. I has 53 protons, 53 electrons, and 78 neutrons: 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10 5s 2 5p 5.⬆️⬆️⬆️ The Electron configuration of Gadolinium is 4f75d16s2 . The name of this element comes from a mineral known as gadolinite, which was given in honor of . The oxide of this element in the isotope 203, i.e. Gd 203, is white, occurs in the form of powder and colorless solutions of its salts.Gadolinium occurs in 6 natural isotopes: 154 Gd, 155 Gd, 156 Gd. 157 Gd, 158 Gd and 160 Gd. . Knowledge of the electron configuration of different atoms is useful in understanding the structure of the periodic table of elements. Every solid, liquid, gas, and plasma is composed of neutral or ionized atoms. The .Gadolinium is a chemical element of the periodic table with chemical symbol Gd and atomic number 64 with an atomic weight of 157.253 u and is classed as a lanthanide. . Electron configuration chart. 1s 2: 2s 2: 2p 6: 3s 2: 3p 6: 3d 10: 4s 2: 4p 6: 4d 10: 4f 7: 5s 2: 5p 6: 5d 1: 6s 2: Electrons per shell: 2, 8, 18, 25, 9, 2: Valence electrons .Gadolinium. Element 64 of Periodic table is Gadolinium with atomic number 64, atomic weight 157.25. Gadolinium, symbol Gd, has a Simple Hexagonal structure and Silver color. Gadolinium is a Lanthanide element. It is part of group null (). Know everything about Gadolinium Facts, Physical Properties, Chemical Properties, Electronic configuration . The third major category of elements arises when the distinguishing electron occupies an f subshell. The first example occurs in the case of the lanthanoids (elements having atomic numbers between 57 and 71).The lanthanoids have the general electron configuration [Kr]4d 10 4f i 5s 2 5p 6 5d 0 or 1 6s 2. where i is a number .
The electronic configurations of Eu (Atomic no. 63), Gd (Atomic No. 64) and Tb (Atomic No. 65) are: Join BYJU'S Learning Program Grade/Exam 1st Grade 2nd Grade 3rd Grade 4th Grade 5th Grade 6th grade 7th grade 8th Grade .
The simple answer: The elements will lie in the s, p, d or f block will completely depend upon the subshell in which the last electron will enter. For example; the electron configuration of gadolinium is . Verified. 29.1k + views. Hint: (1) Electronic configuration is a representation of the distribution of electrons in an atom. Gd or gadolinium is an element of the Lanthanide series, i.e., it is an f-block element. An f-block element has partially filled subshell of the third to the outermost energy shells in its elementary or ionic state.Iba pa The electron configuration for Gd (gadolinium) is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10 4f 7 5s 2 5p 6 5d 1 6s 2. 4. How is electron configuration determined? Electron configuration is determined by the atomic number of an element, which indicates the number of protons in the nucleus. The electrons are then distributed into energy .Gadolinium is a chemical element; it has symbol Gd and atomic number 64. Gadolinium is a silvery-white metal when oxidation is removed. . Its 64 electrons are arranged in the configuration of [Xe]4f 7 5d 1 6s 2, of which the ten 4f, 5d, and 6s electrons are valence.
The electronic configuration of Gd 2+ is (at. No. of Gd is 64) View Solution. Q3. The electronic configuration of gadolinium (Atomic number 64) is (a) [X e] 4 f 3 5 d 5 6 s 2 (b) [X e] 4 f 7 5 d 2 6 s 1 (c) [X e] 4 f 7 5 d 1 6 s 2 (d) [X e] 4 f 8 5 d 6 6 s 2. View Solution. Q4. An element with the electronic configuration [X e] 4 f 7 5 d 1 6 s .The outer electronic configuration of Gd (Atomic No.: 64) is. Q. The outer electron configuration of Gd (Atomic No. 64) is : Q. The outer shell electronic configuration of Gd(Z=64) is. Q. The electronic configuration of Gd2+ is (atomic number of Gd is 64) : Q.
The electronic configuration of Gd 2+ is (at. No. of Gd is 64) View Solution. Q3. The electronic configuration of gadolinium (Atomic number 64) is (a) [X e] 4 f 3 5 d 5 6 s 2 (b) [X e] 4 f 7 5 d 2 6 s 1 (c) [X e] 4 f 7 5 d 1 6 s 2 (d) [X e] 4 f 8 5 d 6 6 s 2. View Solution. Q4. Gadolinium (Gd) has a 4 f 7 5 d 1 6 s 2 electronic configuration .
Gd – 3e – → Gd 3+ Here, the electron configuration of gadolinium ion is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10 4f 7 5s 2 5p 6. This gadolinium ion(Gd 3+) has sixty-four protons, . Now, the electron configuration of gadolinium shows that the last shell of gadolinium has two electrons and the 4f and 5d orbital has a total of eight .
The use of Florida International University's information technology resources is contingent upon proper authorization. By logging in to this system, you agree to abide by all applicable federal, state and local laws, State of Florida Board of Governors rules, and University rules, regulations and policies.
electron configuration of gd|Iba pa